Three Complete Responses in Azer-Cel Allogeneic CD19 CAR T Phase 1b Trial in Blood Cancer (Diffuse Large B-Cell Lymphoma)
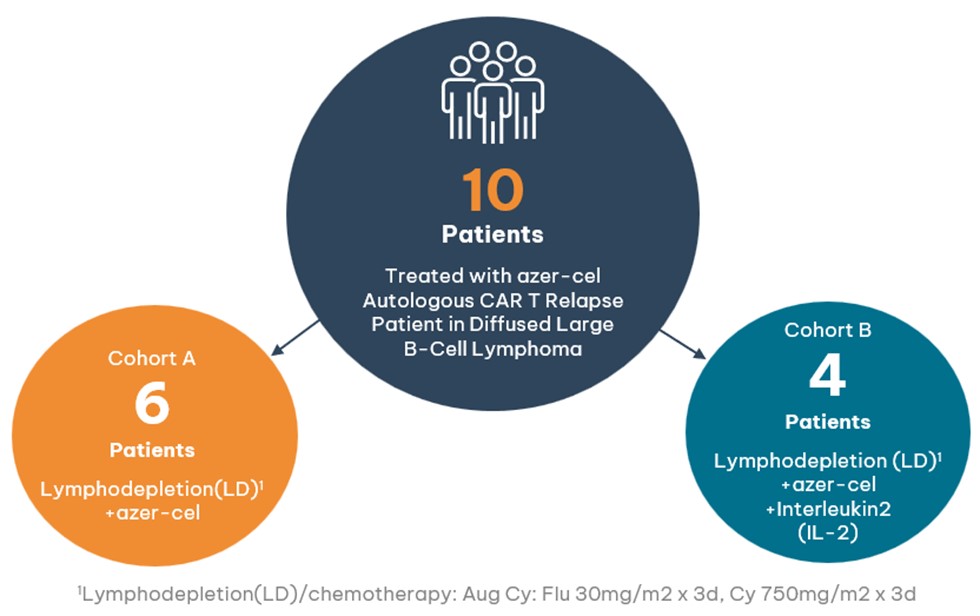
- 10 patients have been treated to date with azer-cel in the Phase 1b diffuse large B-cell lymphoma (DLBCL) trial
- Cohort A: 6 patients were treated with azer-cel and lymphodepletion (chemotherapy)1
- Cohort B: 4 patients were treated with azer-cel, lymphodepletion (chemotherapy)1, and interleukin 2 (IL-2)
- 3 complete responses - The first 2 patients treated in Cohort B achieved a complete response (CR) and 1 patient treated in Cohort A achieved a CR
- Durability of CRs in Cohort B; >120 days and >90 days; all patients ongoing
- All 4 patients in Cohort B are currently ongoing in the trial with 1 patient still awaiting evaluation, additional patients to be enrolled into this cohort
- All patients in the trial had failed multiple prior treatments (4-5 prior lines of therapy), including autologous CAR T therapies
- Treatment with azer-cel to date has been safe and tolerable
- Cohort B regimen is planned to be included in the potential Registrational FDA Phase 2/3 package
SYDNEY, Sept. 03, 2024 (GLOBE NEWSWIRE) -- Imugene Limited (ASX:IMU), a clinical stage immuno-oncology company, is pleased to announce promising results from its Phase 1b clinical trial with azer-cel (azercabtagene zapreleucel, an allogeneic off-the-shelf CD19 CAR T), in patients with relapsed/refractory diffuse large B cell lymphoma (DLBCL), a type of non-Hodgkin’s lymphoma (NHL). All enrolled patients had cancer that had returned following autologous CAR T therapy, a high unmet need for this patient population.
“We are delighted that the first 2 patients in the Cohort B in our azer-cel Phase 1b trial achieved a complete response and continue to maintain their complete responses, one for over 120 days and the other for over 90 days," said Paul Woodard, MD, Imugene’s Chief Medical Officer. “All four patients enrolled in Cohort B have failed 4 to 5 prior treatments, including autologous CAR T therapy. All 4 patients remain on the study and given the robust response rates and durability seen to date, we will continue to enrol patients in the azer-cel plus IL-2 cohort and will closely follow all patients for further responses and durability.”
Cohorts A and B
Patients in the trial are being recruited across 15 leading cancer centres in the U.S. including, Columbia University, University of Minnesota, Emory, and Moffitt Cancer Centres and plans are ongoing to open up to 5 sites in Australia.
Results:
Evaluable: patients who qualify for at least their first 28-day scan
Overall response rate (ORR): the percentage of people in the study who have a complete response (CR) or partial response (PR) to the treatment as confirmed by scan
Responses: a complete response (CR) is defined as disappearance of all tumours; a partial response (PR) is defined as a reduction in tumours as confirmed by scan
Durability of Response (DOR): how long responses last
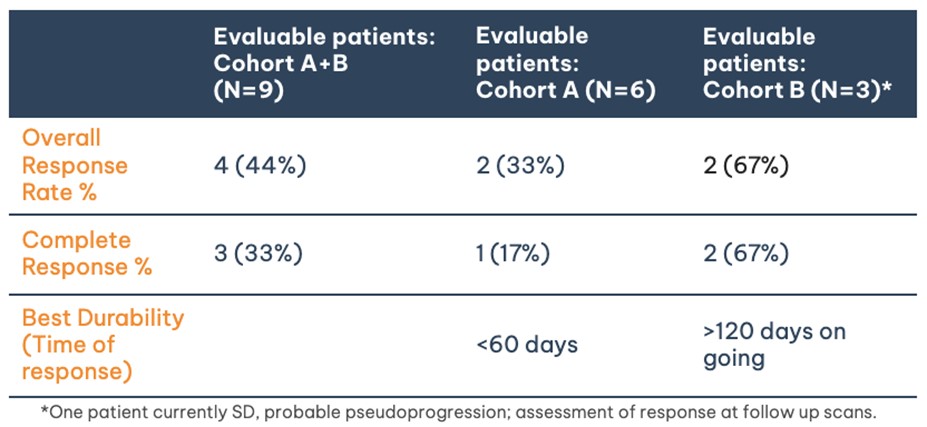
Nine (9) patients total from Cohorts A and B are considered evaluable (qualified for at least day 28 scan). One (1) patient (Cohort B) has been treated and is awaiting their 28-day scan:
- Of the 6 evaluable patients in Cohort A:
- 1 CR, 1 PR = 33% Overall Response Rate (ORR)
- 1 CR = 17% CR
- Durability of response was < 60 days
- All patients no longer on trial
- Of the 3 evaluable patients in Cohort B:
- 2 CRs = 67% ORR
- 2 CRs = 67% CR
- 1 Stable Disease (SD)*: On PET/CT scan imaging, patient’s tumour has decreased however, due to potential T-cell infiltration, noted an increase in signal intensity. This could represent pseudoprogression. The patient remains on trial and continues to be assessed for response at the follow up scans.
- Durability of response thus far: >120 days and >90 days (all patients are ongoing)
- All 4 patients (including 1 patient awaiting 28-day scan) continue on trial
“I am proud of our clinical development team who assessed ways to enhance azer-cel's durability of response, as one of the biggest challenges in CAR T therapy is ensuring that the modified T-cells stay in the body long enough to kill cancer cells,” said Leslie Chong, Managing Director and CEO of Imugene. “To maximise the response rates and durability further, we added a very low dose of IL-2 to the regimen in Cohort B. We are pleased with the results, which suggest improved outcomes in patients, and we look forward to amassing more data using this dosing regimen. We will continue to seek biomarker evidence from Cohort B patients that suggest our strategy is improving the performance of azer-cel.”
The company will continue to enrol additional patients in Cohort B and follow patients for durability of response with the goal of providing a comprehensive package to the FDA for the potential Phase 2/3 registrational trial. Subject to patient recruitment, the company aims to provide an interim Phase 1b data update.
If successful, azer-cel has the potential to become the first approved allogeneic CAR T cell therapy for blood cancer. Beyond studying its efficacy in blood cancers, in the future, Imugene plans to combine azer-cel with its novel onCARlytics program for the treatment of patients with solid tumours, opening a potentially large market for azer-cel in the 90% of cancer not classified as blood cancers.
About the Phase 1b azer-cel trial
The azer-cel allogeneic CAR T trial is an ongoing, open-label, multi-centre Phase 1b clinical trial in the U.S. and Australia, for patients with DLBCL, an aggressive type of non-Hodgkin’s lymphoma (NHL), who relapsed after prior treatment with autologous CAR T therapies. All patients treated with azer-cel and lymphodepletion (LD) demonstrated an acceptable safety profile. In addition, the current patients in Cohort B, treated with azer-cel, LD, and IL-2 are demonstrating clinically meaningful activity and durability.
About diffuse large B cell lymphoma (DLBCL)
DLBCL is an aggressive and fast-growing type of non-Hodgkin’s lymphoma (NHL), a type of blood cancer. DLBCL is the most common type of NHL, with approximately 80,500 cases per year and approximately 30,000 new cases per year in the U.S.2 Relapsed/refractory DLBCL has a high unmet medical need; 60-65% of patients treated with treatments, including autologous CD19 CAR T, relapse.
About Interleukin 2 (IL-2)
IL-2 is a cytokine (a protein that affects what happens between cells in the immune system) that helps T-cells (which are part of the immune system that help fight cancer) grow and survive. IL-2 has been shown to help T cells live longer and to enhance the cancer killing functions of CAR T cells, making them more effective at targeting and killing cancer cells.
References:
1Lymphodepletion (LD)/chemotherapy: Aug Cy: Flu 30mg/m2 x 3d, Cy 750mg/m2 x 3d21NIH
2 NIH National Library of Medicine, PDQ Cancer Information Summaries, May 18, 2023
For more information please contact:
Leslie Chong
Managing Director and Chief Executive Officer
info@imugene.com
Investor Enquiries
shareholderenquiries@imugene.com
Media Enquiries
Matt Wright
matt@nwrcommunications.com.au
U.S Investor Enquiries
Heather Armstrong
harmstrong@imugene.com
Connect with us on LinkedIn @Imugene Limited
Follow us on Twitter @TeamImugene
Watch us on YouTube @ImugeneLimited
About Imugene (ASX:IMU)
Imugene is a clinical stage immuno-oncology company developing a range of new and novel immunotherapies that seek to activate the immune system of cancer patients to treat and eradicate tumours. Our unique platform technologies seek to harness the body’s immune system against tumours, potentially achieving a similar or greater effect than synthetically manufactured monoclonal antibody and other immunotherapies. Our pipeline includes an off-the-shelf (allogeneic) cell therapy CAR T drug azer-cel (azercabtagene zapreleucel) which targets CD19 to treat blood cancers. Our pipeline also includes multiple immunotherapy B-cell vaccine candidates and an oncolytic virotherapy (CF33) aimed at treating a variety of cancers in combination with standard of care drugs and emerging immunotherapies such as CAR T’s for solid tumours. We are supported by a leading team of international cancer experts with extensive experience in developing new cancer therapies with many approved for sale and marketing for global markets.
Our vision is to help transform and improve the treatment of cancer and the lives of the millions of patients who need effective treatments. This vision is backed by a growing body of clinical evidence and peer-reviewed research. Imugene is well funded and resourced, to deliver on its commercial and clinical milestones. Together with leading specialists and medical professionals, we believe Imugene’s immuno-oncology therapies will become foundation treatments for cancer. Our goal is to ensure that Imugene and its shareholders are at the forefront of this rapidly growing global market.
Release authorised by the Managing Director and Chief Executive Officer Imugene Limited.
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/5531400d-7727-422a-bc2e-66ba531036ff
https://www.globenewswire.com/NewsRoom/AttachmentNg/161c3d38-445d-4684-bf7c-d4b6846ffa90

© Copyright Globe Newswire, Inc. All rights reserved. The information contained in this news report may not be published, broadcast or otherwise distributed without the prior written authority of Globe Newswire, Inc.



