Phage Therapies Clinical Trial Pipeline Shows Potential with Active Contributions from 20+ Key Companies | DelveInsight
New York, USA, Jan. 23, 2025 (GLOBE NEWSWIRE) -- Phage Therapies Clinical Trial Pipeline Shows Potential with Active Contributions from 20+ Key Companies | DelveInsight
The phage therapy market is experiencing significant growth due to the increasing prevalence of antibiotic-resistant infections and the growing interest in alternative treatments. Advances in biotechnology and ongoing research are driving innovation in phage-based therapeutics, offering a promising solution to combat multidrug-resistant pathogens. Additionally, regulatory support and rising investments in the development of phage therapies are further fueling market expansion. This market is expected to continue growing as healthcare providers seek effective alternatives to traditional antibiotics.
DelveInsight’s 'Phage Therapies Competitive Landscape 2025' report provides comprehensive global coverage of pipeline phage therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the phage therapies pipeline domain.
Key Takeaways from the Phage Therapies Pipeline Report
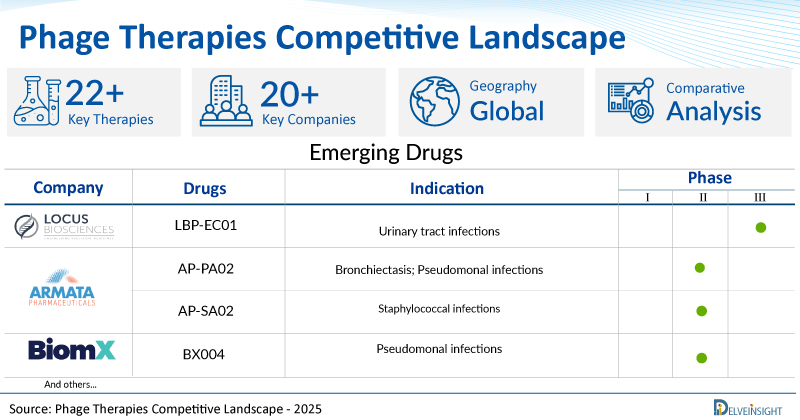
- DelveInsight’s phage therapies competitive report depicts a robust space with 20+ active players working to develop 22+ pipeline phage therapies.
- Key phage therapy companies such as Locus Biosciences, Armata Pharmaceuticals, BiomX, SNIPR Biome, Phagelux, PHAXIAM Therapeutics, Phico Therapeutics, Technophage, and others are evaluating new phage therapies to improve the treatment landscape.
- Promising pipeline phage therapies such as LBP-EC01, AP-PA02, AP-SA02, BX004, SNIPR 001, PL-01-SZ, Research programme: bacteriophage therapies, Research programme: antibacterial protein-based therapeutics, TP-102, and others are under different phases of phage therapies clinical trials.
Request a sample and discover the recent advances in phage therapies drugs @ Phage Therapies Competitive Report
Phage Therapies Overview
Phage therapy is an emerging method for treating bacterial infections using bacteriophages—viruses that specifically infect and destroy bacteria. This approach harnesses the natural ability of phages to target bacteria without damaging human cells, offering a promising alternative to conventional antibiotics. With the rise of antibiotic-resistant bacteria, phage therapy has garnered renewed interest as it presents a potential solution to this growing global health concern. Unlike broad-spectrum antibiotics, which can harm the body’s beneficial bacteria, phages are highly specific to their bacterial targets, reducing collateral damage.
In phage therapy, the phages attach to bacterial cell surfaces, inject their genetic material, and replicate inside the host. This replication process causes the bacteria to burst, releasing new phages that continue the infection cycle until the bacteria are eradicated. Each phage strain typically targets a particular bacterial species or strain, so it is crucial to match the right phage to the infection, which necessitates accurate bacterial identification through diagnostic testing.
Phage therapy offers several advantages, including the ability to combat antibiotic resistance. Since phages can evolve alongside bacteria, they can help prevent the development of long-term resistance. Phage therapy can also be combined with antibiotics to improve treatment outcomes. This combination can help disrupt biofilms, which are protective layers formed by bacteria that antibiotics alone may struggle to penetrate. Additionally, phages are abundant in nature, providing opportunities to discover new strains to tackle emerging bacterial threats.
Phage Therapies Market Dynamics
The phage therapy market has been witnessing significant growth due to the increasing concern over antibiotic resistance and the rise in bacterial infections. Phage therapy, which uses bacteriophages to target and kill specific bacterial pathogens, is being recognized as an alternative to conventional antibiotics. This shift is especially relevant in the context of antimicrobial resistance (AMR), which has rendered many antibiotics ineffective. The market is being propelled by both regulatory advancements and increased funding, which are supporting the development of new phage-based products. As a result, pharmaceutical companies and biotechnology firms are increasingly investing in the research and commercialization of phage therapies.
One of the main drivers of the phage therapy market is the urgent need for novel treatments for multi-drug-resistant (MDR) infections. Hospitals and healthcare systems are facing a growing number of cases where traditional antibiotics no longer offer effective solutions. Phages have shown promise in treating infections caused by resistant strains such as Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. With ongoing research into phage formulations, personalized treatments, and the ability to target specific bacteria without harming the human microbiome, phage therapy is gradually positioning itself as a feasible and effective treatment option for these difficult-to-treat infections.
However, the phage therapy market faces challenges such as regulatory hurdles, public perception, and scientific uncertainties. Regulatory bodies, particularly the FDA and EMA, are still working on defining clear guidelines for the approval of phage-based products. Phages are highly specific in nature, and formulating standardized products that can be widely used presents challenges in terms of scalability and consistency. Moreover, because phages are biological entities, their use raises concerns regarding long-term safety and efficacy, which are not yet fully understood. These challenges may delay broader market adoption and increase the time-to-market for phage-based therapies.
In conclusion, the phage therapy market is poised for significant expansion, driven by the increasing need for alternative treatments to combat antibiotic resistance. Although challenges such as regulatory complexities and scientific uncertainty remain, ongoing innovations, strategic partnerships, and a favorable market environment are likely to position phage therapy as a key player in the future of infectious disease management. The market's success will depend largely on overcoming these hurdles while proving the clinical effectiveness and safety of phage-based treatments.
To know more about phage therapies, visit @ Phage Therapies Market Insights
Phage Therapies Pipeline Analysis: Drug Profile
LBP-EC01: Locus Biosciences
LBP-EC01 is an experimental Locus crPhage therapy being developed for the treatment of urinary tract infections (UTIs) and other infections caused by Escherichia coli. It is a bacteriophage cocktail engineered with a CRISPR-Cas3 construct that targets the E. coli genome. This precision medicine works through a novel dual mechanism, combining the natural lytic action of the bacteriophage with the DNA-targeting activity of CRISPR-Cas3. Laboratory tests and small animal models of UTIs have shown that LBP-EC01 is significantly more effective at killing E. coli than natural bacteriophages. It successfully met all primary and secondary endpoints while demonstrating safety and tolerability in a Phase 1b trial. LBP-EC01 is currently undergoing evaluation in a Phase II/III registrational trial for E. coli-caused UTIs.
AP-PA02: Armata Pharmaceuticals
AP-PA02 is a therapeutic phage cocktail designed to target the P. aeruginosa pathogen to treat severe respiratory infections, particularly in patients with cystic fibrosis and non-cystic fibrosis bronchiectasis (NCFB). This formulation includes a mix of natural P. aeruginosa phages from various families and subfamilies, each targeting different receptor types. The phages work together effectively and cooperatively, demonstrating high potency and a broad host range.
AP-PA02 is produced as a sterile liquid formulation for inhalation delivery. The clinical trial material is manufactured following cGMP standards at Armata’s facility in Marina Del Rey, California. Currently, it is undergoing evaluation in Phase II clinical trials for Bronchiectasis and Pseudomonal infections.
Find out more about phage therapies drugs @ Phage Therapies Analysis
A snapshot of the Pipeline Phage Therapies mentioned in the report:
| Drugs | Company | Phase | Indication |
| LBP-EC01 | Locus Biosciences | II/III | Urinary tract infections |
| AP-PA02 | Armata Pharmaceuticals | II | Bronchiectasis; Pseudomonal infections |
| AP-SA02 | Armata Pharmaceuticals | I/II | Staphylococcal infections |
| BX004 | BiomX | I/II | Pseudomonal infections |
| SNIPR 001 | SNIPR Biome | I | Escherichia coli infections |
Learn more about the emerging phage therapies @ Phage Therapies Clinical Trials
Key Developments in the Phage Therapies Treatment Space
- In December 2024, PHAXIAM Therapeutics a biopharmaceutical company developing innovative treatments for severe and resistant bacterial infections, announced the clinical results of the PhagoDAIR I pilot study, demonstrating an excellent phage safety profile and a 74% infection control rate in the Phages arm for patients who received a single intra-articular injection, consistent with clinical data that observed in patients treated on a compassionate basis.
- In August 2024, Locus Biosciences recently reported positive pharmacokinetic and safety data from the first part of the ongoing Phase II trial of CRISPR-edited phage therapy candidate LBP-EC01. LBP-EC01 is being developed as a novel treatment for uncomplicated urinary tract infections caused by antimicrobial-resistant and multi-drug-resistant E. coli.
- In July 2024, Armata Pharmaceuticals, Inc. announced that it has received an additional $5.25 million of non-dilutive funding under a previously announced Department of Defense grant, received through the Medical Technology Enterprise Consortium (MTEC) and managed by the Naval Medical Research Command (NMRC) – Naval Advanced Medical Development (NAMD) with funding from the Defense Health Agency and Joint Warfighter Medical Research Program. The grant was awarded to Armata to support the clinical development of its optimized phage candidate, AP-SA02, as a potential treatment for complicated Staphylococcus aureus bacteremia.
- In March 2024, BiomX Inc., a clinical-stage company advancing novel natural and engineered phage therapies that target specific pathogenic bacteria, announced the acquisition of Adaptive Phage Therapeutics, Inc., a U.S.-based privately-held, clinical-stage biotechnology company pioneering the development of phage-based therapies to combat bacterial infections, and its previously announced $50 million private placement to certain institutional accredited investors, which was led by affiliates of Deerfield Management Company and the AMR Action Fund, and additional investors including the Cystic Fibrosis Foundation, OrbiMed and Nantahala Capital.
Scope of the Phage Therapies Pipeline Report
- Coverage: Global
- Key Phage Therapies Companies: Locus Biosciences, Armata Pharmaceuticals, BiomX, SNIPR Biome, Phagelux, PHAXIAM Therapeutics, Phico Therapeutics, Technophage, and others
- Key Phage Therapies Pipeline Therapies: LBP-EC01, AP-PA02, AP-SA02, BX004, SNIPR 001, PL-01-SZ, Research programme: bacteriophage therapies, Research programme: antibacterial protein-based therapeutics, TP-102, and others
Dive deep into rich insights for new phage therapy treatments, visit @ Phage Therapies Drugs
Table of Contents
| 1. | Phage Therapies Pipeline Report Introduction |
| 2. | Phage Therapies Pipeline Report Executive Summary |
| 3. | Phage Therapies Pipeline: Overview |
| 4. | Phage Therapies Marketed Drugs |
| 5. | Phage Therapies Clinical Trial Therapeutics |
| 6. | Phage Therapies Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Phage Therapies Pipeline: Late-Stage Products (Phase III) |
| 8. | Phage Therapies Pipeline: Mid-Stage Products (Phase II/III) |
| 8.1. | LBP-EC01: Locus Biosciences |
| 9. | Phage Therapies Pipeline: Early-Stage Products (Phase I) |
| 9.1. | SNIPR 001: SNIPR Biome |
| 10. | Phage Therapies Pipeline: Preclinical and Discovery Stage Products |
| 11. | Phage Therapies Pipeline Therapeutics Assessment |
| 12. | Inactive Products in the Phage Therapies Pipeline |
| 13. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 14. | Unmet Needs |
| 15. | Phage Therapies Market Drivers and Barriers |
| 16. | Appendix |
For further information on the phage therapies pipeline therapeutics, reach out @ Phage Therapies Therapeutics
Related Reports
Urinary Tract Infections Pipeline
Urinary Tract Infections Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key UTIs companies, including Wockhardt, GlaxoSmithKline, Iterum Therapeutics, Spero Therapeutics, VenatoRx Pharmaceuticals, Helperby Therapeutics, Spexis, LUCA Biologics, Seed Health, Inc., Aelin Therapeutics, Omnix Medical, Inmunotek S.L., Sinovent Pty Ltd., Qilu Pharmaceuticals, Entasis Therapeutics, Adaptive Phage Therapeutics, Locus Biosciences, Nabriva Therapeutics, Utility Therapeutics, Zensun (Shanghai) Sci & Tech, Fedora Pharmaceuticals, Osel Inc., Evofem Biosciences, Enlivex, Fimbrion Therapeutics, Rebiotix, MerLion Pharmaceuticals, Allecra Therapeutics, Lakewood Amedex, Sumitomo Dainippon Pharma, Sihuan Pharmaceuticals, Super Trans Medical, Asieris Pharmaceuticals, among others.
Uncomplicated Urinary Tract Infection Market
Uncomplicated Urinary Tract Infection Market Insight, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key uncomplicated UTI companies, including GlaxoSmithKline, Iterum Therapeutics, Inmunotek, Janssen Pharmaceuticals, Fimbrion Therapeutics, among others.
Complicated Urinary Tract Infection Market
Complicated Urinary Tract Infection Market Insight, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key complicated UTI companies, including Spero Therapeutics, Wockhardt, Venatorx Pharmaceuticals, Allecra Therapeutics, Nabriva Therapeutics AG, MerLion Pharmaceuticals, among others.
Complicated Urinary Tract Infection Pipeline
Complicated Urinary Tract Infection Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key cUTIs companies, including Spero Therapeutics, Iterum Therapeutics, Venatorx Pharmaceuticals, Inc., Nabriva Therapeutics, Allecra Therapeutics, Wockhardt, MerLion Pharmaceuticals, among others.
Urinary Tract Obstruction Treatment Devices Market
Urinary Tract Obstruction Treatment Devices Market Insight, Competitive Landscape, and Market Forecast – 2030 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key urinary tract obstruction treatment devices companies, including B. Braun Melsungen AG, Boston Scientific Corporation, Cook, Becton, Dickinson, and Company, Teleflex Incorporated, Olympus, Dornier Medtech, EMS Urology, Advin Health Care, Inceler Medikal Co. Ltd., STORZ MEDICAL AG, Shockwave Medical Inc., ACE Medical Devices Pvt. Ltd., Walz Elektronik GmbH, Applied Medical Resources Corporation, Richard Wolf GmbH, Stryker, Urocare Products, Inc., J and M Urinary Catheters LLC, Rocamed, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
© Copyright Globe Newswire, Inc. All rights reserved. The information contained in this news report may not be published, broadcast or otherwise distributed without the prior written authority of Globe Newswire, Inc.


